The new version 10 of BAYOOSOFT Risk Manager
The BAYOOSOFT Risk Manager is now available to all customers and interested parties in the latest version 10.0. With this release, the Risk Manager is extended by the Clinical Evaluation module, which supports you in the process of the same name and enables you to start directly with the identification and verification of relevant data.
Additional enhancements are:
- The change of name from Qware® Riskmanager to BAYOOSOFT Risk Manager announced in November 2019 finds its way into the user interface of the solution with this version.
- The page Post Production Reminder provides a simple overview of all open post production reminders across projects and versions and allows you to open them directly for editing as well.
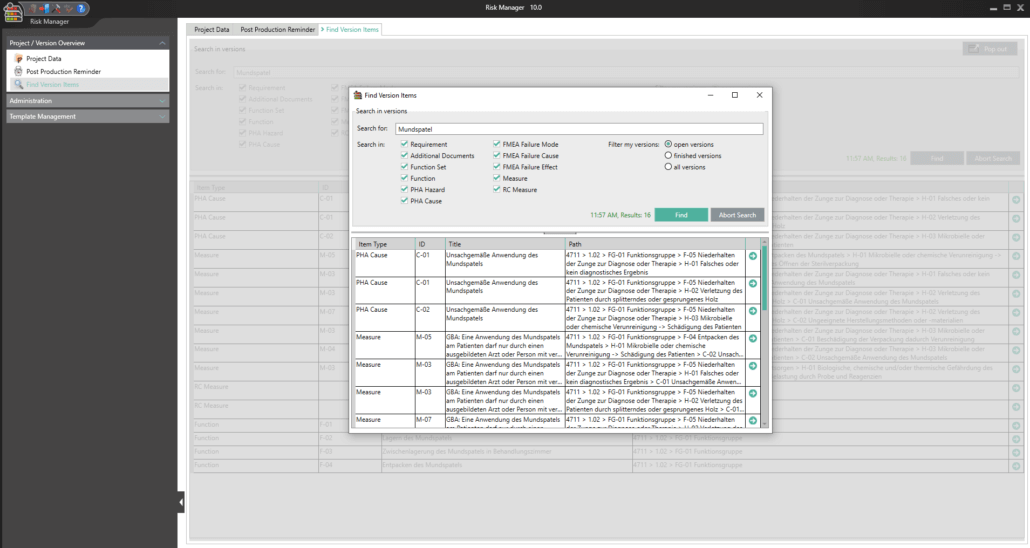
- In order to enable a easy search for version items across projects and versions a separate search function has been added which can fold out as pop-up window to search and edit the items at the same time.
- For the checklist modules MEE (Medica Electrical Equipment) and ERQ (Essential Requirements) an automated negation text will be added, when requirements are filtered as not relevant for the medical device manufacturer.