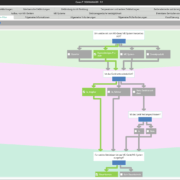
Accelerate the clinical evaluation process with tool support
Digital tools can support manufacturers to centrally map the clinical evaluation according to the Medical Devices Regulation MDR 2017/745, MDCG & MEDDEV 2.7/1 rev 4, to standardise procedures and to document them comprehensibly. This makes quality management faster and more efficient, while redundancies are avoided and symbioses are used.
The practical lecture gives an overview of how a clinical evaluation can be completely covered and accelerated with tool support.