Good to know
When answering individual questions, you will be supported by a compilation of the implementation regulations to be observed which can be displayed. In this way, you can reach the classification result of your IVD as quickly and conveniently as possible. After determining the classification result, you can have the corresponding report output in the documentation area.


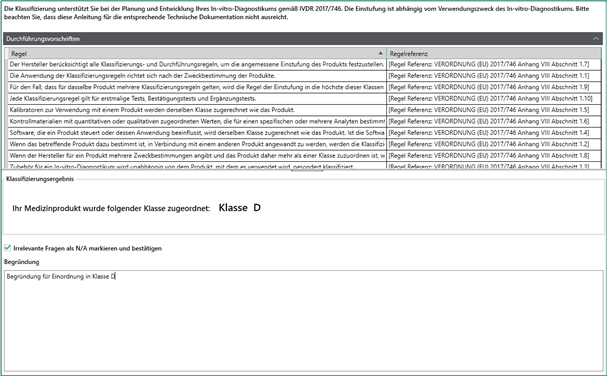
 Photo by Dana Tentis from Pexels
Photo by Dana Tentis from Pexels